Ammonia is a common chemical compound used as a fertilizer, cleaner, and in various other applications such as metallurgical processes, such as nitriding alloy sheets, which harden metal surfaces. It is an essential raw material for the production of nitric acid, ammonium salts and amines, it also can be easily converted into hydrogen, which is used in welding and other processes. Ammonia can also absorb large amounts of heat, making it an excellent choice for cooling applications, such as in air-conditioning and refrigeration equipment. However, ammonium hydroxide is very toxic, exposure to the compound can cause severe burns to the eyes and skin, as well as respiratory tract irritation. It can be fatal when breathed in large quantities. However, it is highly corrosive to metals, it is compatible with carbon steel, iron, stainless steel, titanium alloy, and aluminum alloys but is not compatible with copper, copper get into a blue-green salt if it is exposed to it. How did it happen?
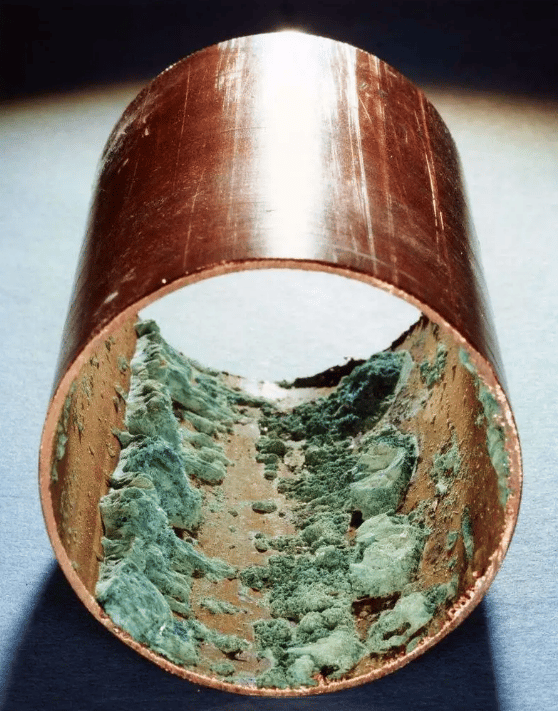
- Copper and zinc alloys (brass), including admiralty brass and Al-Cu, are prone to cracking; The zinc content of brass affects the sensitivity, especially when the mass fraction of Zn exceeds 15%.
- An aqueous solution of NH3 or ammonium compound, and aerobic.
- PH higher than 8.5.
Other environments that can cause stress corrosion of copper alloys include air, fresh water and seawater seriously polluted by SO2; Sulfuric acid, nitric acid, steam, tartaric acid, acetic acid, citric acid and other aqueous solutions, ammonia and mercury for cleaning parts. Where liquid ammonia (water content not exceeding 0.2%) may be contaminated by air (oxygen or carbon dioxide); In fact, oxygen and other oxidizing agents such as water are important conditions for stress corrosion of copper. During processing, petroleum refining has a lot of potential corrosion due to impurities in crude oil and additives. Types of cracking corrosion caused by ammonia include:
H2S-NH3-H2O Corrosion
It depends on the concentration, velocity and characteristics of the medium. The higher the concentration of NH3 and H2S, the more serious the corrosion. The higher the fluid velocity in the copper pipe, the stronger the corrosion. Low flow rates lead to ammonium deposition and localized corrosion. Some media (such as cyanide) may exacerbate corrosion, while oxygen (along with the injected water) may accelerate corrosion.
Ammonia corrosion at the top of sulphuric acid alkylation tower
Alkali washing and washing reactor products are important for removing acidic impurities in order to control excessive corrosion in the fractionation zone tower top system. To reduce the corrosion rate and reduce the amount of inhibitor used, neutralizing amine or NH3 can neutralize the tower top water condensate to a pH of 6 to 7. However, in some cases, NH3 can cause stress corrosion cracking of navy brass tubes in overhead condensers.
Ammonia corrosion in catalytic reforming plant
Stress corrosion cracking caused by ammonia is one of several types of stress corrosion cracking in catalytic reforming units. NH3 exists in the effluent of the pretreatment reactor and reforming reactor and dissolves in water to form ammonia, resulting in rapid stress corrosion cracking of CU-based alloys.
Ammonia corrosion in the delayed coking unit
The equipment of delayed coking units is susceptible to low-temperature corrosion mechanisms, including stress cracking of copper based alloys caused by ammonia. These corrosion mechanisms play a role in water quenching, steam coke cleaning and venting. However, all coking towers usually have exhaust pipes and tanks that are exposed almost continuously to moist exhaust vapors and liquids. The vapors and liquids formed and discharged by sudden cooling usually contain large amounts of H2S, NH3, NH4Cl, NH4HS and cyanide, which are released from the thermal cracking reaction of the feed to the coking plant. Due to the presence of NH3 in coking units, stress corrosion cracking caused by ammonia can occur in copper alloy tubes with high pH values.
Ammonia corrosion in sulfur recovery units
The gas feed is usually rich in H2S and saturated water vapor, and may also be mixed with hydrocarbons and amines, which may cause H to permeate the metal, where gases from hydrogen-induced cracking (including hydrogen bulging) and sulfide stress cracking (SSC) may be present. In addition, NH3 may be present in the gas feed, leading to stress corrosion cracking, while cyanide can also speed up corrosion.
Brass containing less than 20% zinc generally does not suffer stress corrosion cracking in the natural environment. The higher the zinc content of brass is, the greater the stress corrosion fracture sensitivity is. Adding aluminum, nickel and tin to brass can reduce stress corrosion cracking. When the Zn content is lower than 15%, the corrosion resistance of Cu-Zn alloy is improved.
Stress elimination by annealing is the most commonly used effective measure to prevent stress corrosion cracking of brass. SCC in the vapor environment can sometimes be controlled by preventing air entry, the sensitivity of copper alloys needs to be assessed by checking and monitoring the PH of water samples and NH3 from time to time, and current inspection or visual inspection of eddy currents can be used to determine whether the heat exchanger beam is broken. But in short, copper and its alloys should be avoided in the production of ammonia and liquid ammonia.







